Our Science
SKIN MICROBIOME
The human skin is home to many types of bacteria, fungi, and viruses that compose the skin microbiome. These 1,000s of different organisms and species have an important role in protecting from pathogens, influences immune functions and the ability of the skin to form a protective barrier, which in turn either promote or disrupt human health (1).
In some disease states, an altered balance of the skin microbiome can occur, a condition known as dysbiosis. This state of dysbiosis contributes to the disruption of immune homeostasis and worsens disease symptoms (1).
For example, atopic dermatitis is often characterized by dysbiosis of the skin microbiome, with an over-abundance of Staphylococcus aureus bacteria (2).
Our work has discovered mechanisms for how selected bacteria cause or prevent diseases and has shown that reintroducing specifically selected beneficial bacterial strains from healthy skin can alleviate disease symptoms as well as kill or inhibit harmful species (3).
Discovery Platform
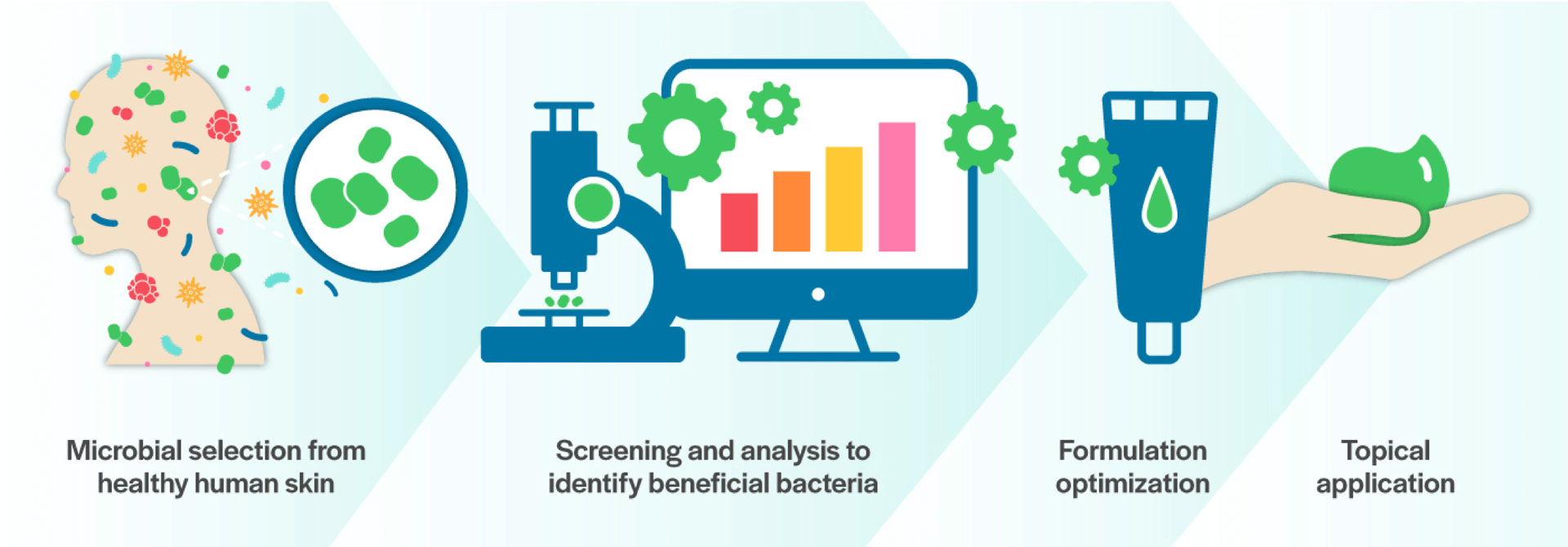
Using the discovery platform based on leading research from the Gallo Laboratory at the University of California San Diego, MatriSys is able to screen thousands of microbes on the skin to identify beneficial bacteria that can treat skin disorders and restore homeostasis to the skin microbiome.
The team at Matrisys then optimizes the beneficial bacteria with the goal to identify and clinically develop a topical prescription formulation.
Groundbreaking Deep Pipeline
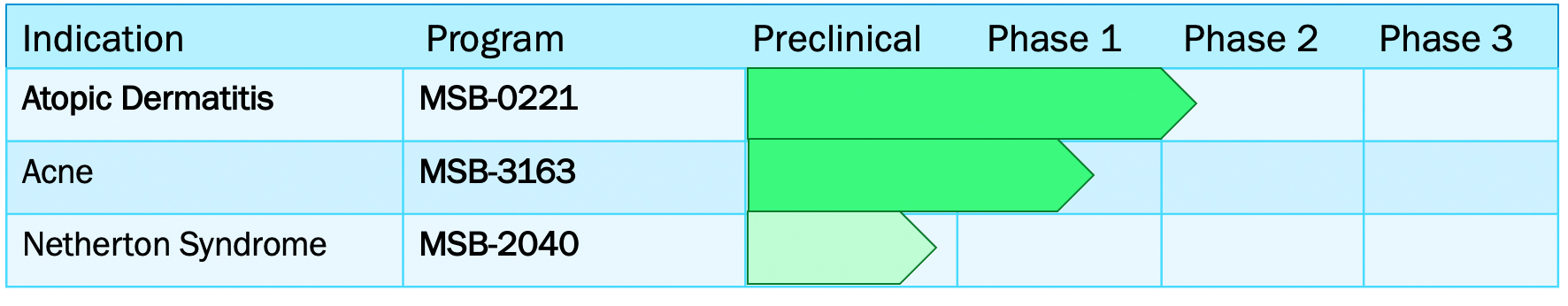
MatriSys leverages a deep clinical pipeline to treat the millions of children and adults suffering from difficult to treat chronic skin diseases.
Applications
Atopic dermatitis (AD) is a chronic inflammatory skin disease which affects up to 20% of children and up to 3% of adults worldwide, posing a significant burden on patients’ quality of life. AD is frequently characterized by overgrowth of Staphylococcus aureus (S. aureus), which then triggers proteolytic breakdown of the epidermal barrier and immune dysregulation.
Existing therapies for AD include drugs that are associated with skin thinning and other safety concerns. Correcting the skin microbial ecosystem with targeted bacteriotherapy is a novel approach that avoids these adverse reactions. Use of a bacterium selected from healthy human skin to restore normal flora breaks the S. aureus colonization cycle and improves skin immune and barrier dysfunction characteristic of AD.
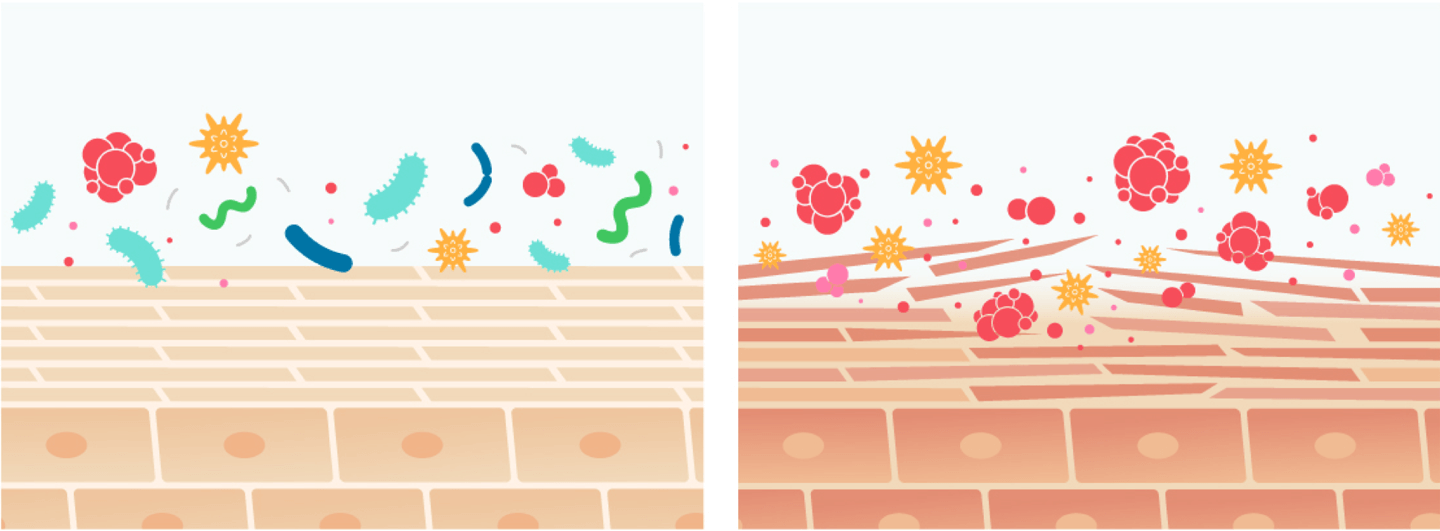
• Balanced and diverse microbiome
• Normal, protective skin barrier
• Microbiome dysbiosis
• Defective, vulnerable skin barrier
Acne
Acne is one of the most common skin diseases, affecting 85% of adolescents and young adults worldwide, resulting in major health care costs. Severe manifestations of acne are painful and cause disfiguration and scarring, and in some patients, profoundly reduces self-esteem and affects mental health.
Host-microbiome interactions that affect both innate and adaptive immune homeostasis appear to be a central factor in this disease, with recent observations suggesting that the composition and activities of the microbiota in acne is perturbed. Cutibacterium acnes is one of the most common bacterial species on human skin and is associated with acne vulgaris.
Existing therapies for acne often use pharmaceutical antibiotics. Overuse of antibiotics promotes dangerous antibiotic resistance in pathogens. Use of targeted bacteriotherapy for acne avoids this problem by using the healthy skin microbiome to control Cutibacterium acnes (6).
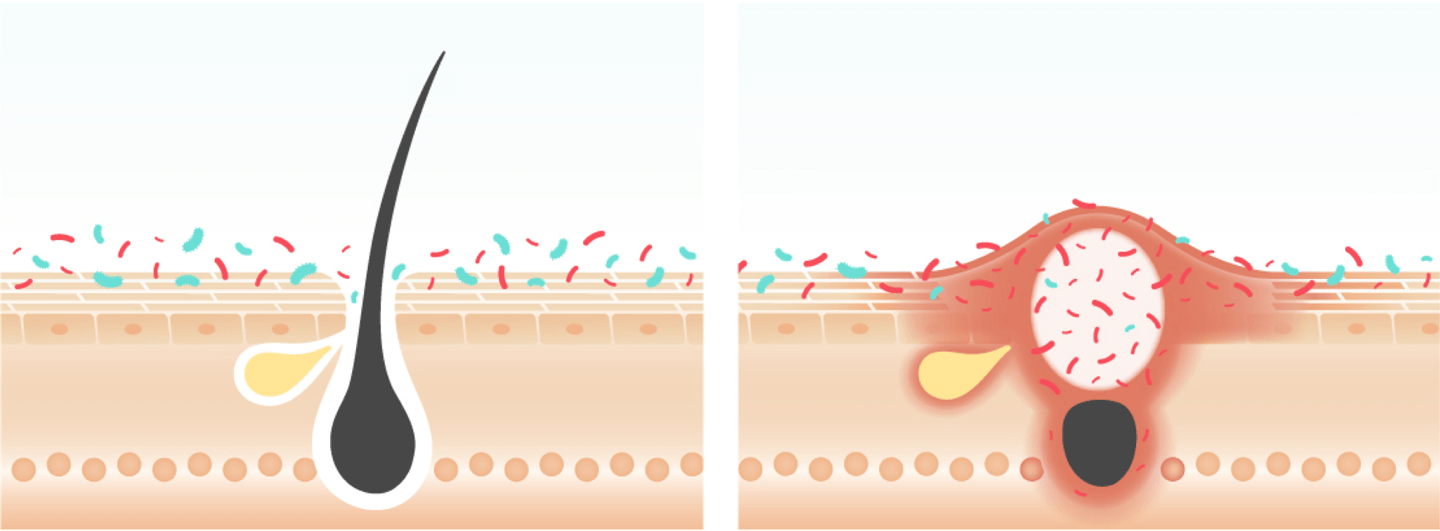
• Balanced microbiome
• Healthy hair follicle
• Bacteria overproliferation
• Inflammation at swollen pore
Netherton Syndrome is a rare human genetic disorder that affects the skin, hair and immune system. The disease is caused by a mutation in the SPINK5 gene. Newborn infants with this disorder have a severe defect in the ability of their skin to form a normal barrier and are at risk of severe dehydration and infections.
The signs and symptoms of disease in Netherton Syndrome improves with age, yet typically fluctuates throughout life. These individuals continue to have recurrent bouts of red, itchy and scaling skin that is very difficult to treat. Because of the nature of this disorder, current drugs for inflammatory skin conditions have to be used with caution.
Recent studies have shown that the severity of disease in adults with Netherton Syndrome is associated with abnormalities caused by the skin microbiome (5). Technology derived from understanding the interaction of the microbiome with skin barrier can target this problem and may improve symptoms associated with this disease.
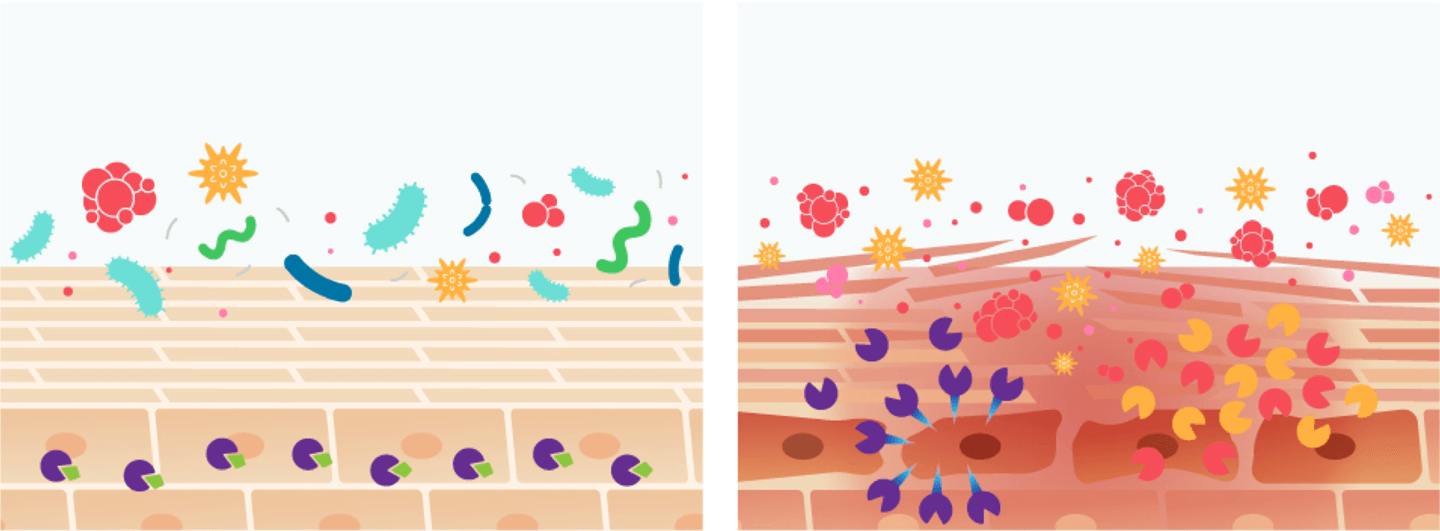
• Proteolytic homeostasis
• Regulated epidermal barrier
• Unregulated protease response
• Epidermal barrier damage and inflammation
MSB-0221
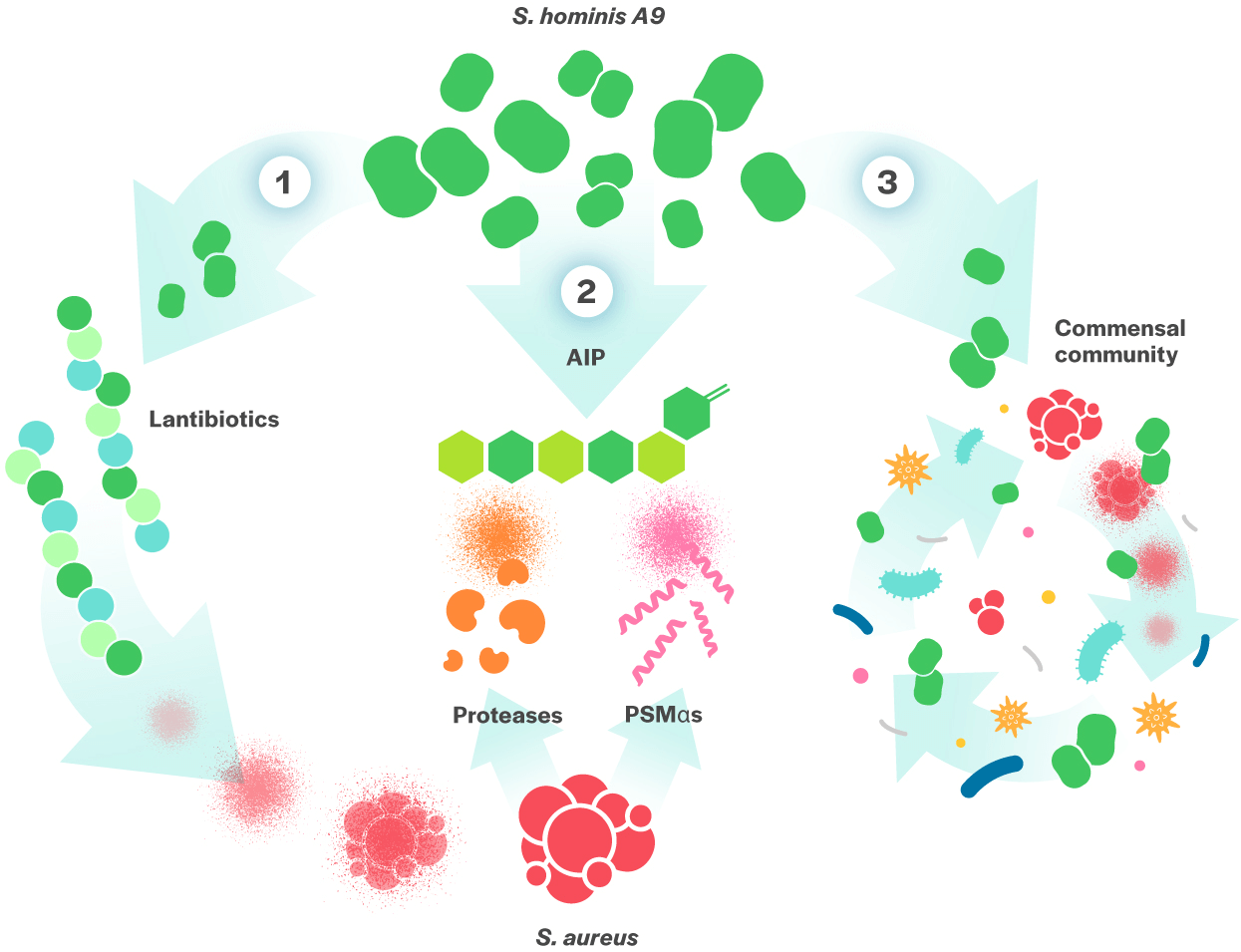
Our lead clinical development program, MSB-0221, is for the treatment of Atopic Dermatitis (AD), a common skin condition which affects more than 18 million patients in the US alone. Current therapies include moisturizers, topical corticosteroids, anti-inflammatory agents (PED4i) and immunosuppressants (topical and systemic), but can be associated with skin thinning and other safety concerns. Given the existing high unmet medical need, new therapies are needed to address the root cause of the disease. The mechanisms by which MSB-0221 may help AD is depicted in the cartoon below.

Antimicrobial activity via production of 2 lantibiotics that directly kill S. aureus.

AIP (autoinducing peptides) production inhibiting the aggressive proteases and PSMs produced by S. aureus, as well as the damaging proteases and toxins from S. epidermis, which degrade the skin barrier and trigger an immune reaction.

Helps the commensal bacterial community recreate itself, preventing further S. aureus, colonization, and has potential to directly act on human skin to improve its defense and decrease inflammation.
- Nakatsuji, T., Chen, T. H., Narala, S., Chun, K. A., Two, A. M., Yun, T., … & Gallo, R. L. (2017). Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Science Translational Medicine, 9(378). [Read More]
- Nakatsuji, T., & Gallo, R. L. (2019). The role of the skin microbiome in atopic dermatitis. Annals of Allergy, Asthma & Immunology, 122(3), 263-269. [Read More]
- Nakatsuji, T., Hata, T. R., Tong, Y., Cheng, J. Y., Shafiq, F., Butcher, A. M., … & Gallo, R. L. (2021). Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine, 1-10. [Read More]
- Nakatsuji T., Gallo R.L., Shafiq F., Tong Y., Chun K., Butcher A.M., … Hata TR (2021). Use of Autologous Bacteriotherapy to Treat Staphylococcus aureus in Patients With Atopic Dermatitis: A Randomized Double- blind Clinical Trial. JAMA Dermatology 157(8)978-982 [Read More]
- Williams, M.R., Cau, L., Wang, Y., Kaul, D., Sanford, J.A.,…..Gallo, R.L. (2020). Interplay of Staphylococcal and Host Proteases Promotes Skin Barrier Disruption in Netherton Syndrome. Cell Reports, 30(9):2923-2933. [Read More]
- O’Neill, A. M., & Gallo, R. L. (2018). Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome, 6(1), 1-16. [Read More]
- Yamasaki, K., & Gallo, R. L. (2011, December). Rosacea as a disease of cathelicidins and skin innate immunity. In Journal of Investigative Dermatology Symposium Proceedings (Vol. 15, No. 1, pp. 12-15). Elsevier. [Read More]
- Yamasaki, K., Di Nardo, A., Bardan, A., Murakami, M., Ohtake, T., Coda, A., … & Gallo, R. L. (2007). Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nature Medicine, 13(8), 975-980. [Read More]
